A guide for families
In anticipation of new opportunities for adults with Down syndrome to participate in clinical trials for Alzheimer’s therapies, LuMind IDSC developed a guide introducing families to the differences in the therapeutic approaches. Download the full guide here, or read on…

People with Down syndrome face a heightened risk of developing Alzheimer’s disease (AD) due to their unique genetic makeup. That makes them more vulnerable to early-onset AD and significantly increases their likelihood of developing AD in their lifetime, compared to the general population. While researchers have made progress in understanding the biological mechanisms underlying Alzheimer’s, much remains to be learned about how to intervene effectively to protect brain health in this community. Today, multiple research efforts are underway to identify effective therapeutic approaches for individuals with Down syndrome, each targeting the problem in a distinct and specialized way.
Understanding the differences among these new therapies is crucial—not only in terms of how they are meant to prevent or slow the progression of AD, but also in the drug delivery methods. Some therapies aim to address the problem at its source, while others focus on clearing existing damage. By exploring these nuances, caregivers and self-advocates can better appreciate the research underway and the steps scientists are taking to potentially improve outcomes for individuals with Down syndrome.
 The answer can be found in the genetic makeup of most people with Down syndrome.
The answer can be found in the genetic makeup of most people with Down syndrome.
Every cell in the human body has a set of instructions (“genes”) that tell cells how to make proteins—those tiny building blocks that help our bodies function properly. People with Down syndrome have an extra copy of a particular gene called the APP gene (illustrated by the blue helix at right), which gives their bodies an extra set of instructions for making a protein called amyloid precursor protein (APP), a protein made in the brain that helps cells function normally.
Imagine a factory that typically handles two orders for APP, producing just enough to meet the body’s needs. In people with Down syndrome, the factory gets three orders instead of two, causing it to overproduce APP—like an assembly line working overtime and creating a surplus of product (in blue in illustration at right).
After APP is made, it naturally breaks down into smaller pieces. Some of those pieces are called amyloid beta (Aß, shown in red in the illustration below). In the brains of people without Down syndrome, extra or used Aß typically gets cleared away by the brain’s natural cleanup systems. However, in the brains of people with Down syndrome, the overproduction of APP protein means that extra Aß is made starting at birth, which can overwhelm these brain cleanup systems.
It’s like the factory’s assembly line is running so fast that too many parts pile up, and the janitors (the brain’s cleanup system) can’t keep up with the extra Aß . Some of these extra Aß pieces can misfold and begin to stick together into clumps (shown in red, below). Once these clumps form, they act like sticky residue on the factory floor, making it harder for normal operations to continue.
The initial Aß clumps can grow into larger clumps that can eventually be seen by a microscope. The larger clumps are called Aß plaques (shown in red, below), which can start to build up around neurons, the cells in the brain that carry our thoughts and store our memories (shown in lavender, below). When smaller clumps and plaques build up in the brain, they can start to interfere with memory and thinking, similar to how a factory with cluttered assembly lines and blocked pathways can’t function effectively. This blockage can lead eventually to Alzheimer’s disease.
People with Down syndrome are more prone to Aß clumping, which is why they have a >90% lifetime risk of developing Alzheimer’s dementia. As such, scientists are working hard to find ways to prevent these Aß clumps and plaques from forming or to remove them after they accumulate, in order to protect their brain health.

Researchers are currently investigating at least three types of therapies that address this buildup of Aß plaques. Each therapy seeks to intervene at different points in the plaque accumulation process, and each involves a different mechanism of intervention.
Antisense Oligonucleotides (ASOs)
Imagine a supervisor stepping in to slow down the factory’s production line by cancelling the extra APP order. ASOs work at the genetic level to reduce the amount of APP protein produced, which is intended to lower the amount of Aß made. This subsequently may prevent excess Aß clumping and reduce plaque levels, which together may slow down disease progression.
To return to our analogy, the ASO helps ensure the factory doesn’t generate an overwhelming surplus of components that could lead to assembly line clutter.
The HERO study, sponsored by Ionis Pharmaceuticals, is an active clinical trial studying the safety and tolerability of an ASO intervention. The mechanism Ionis is testing is administered into the spine (intrathecal), by lumbar puncture, which allows the ASO to be delivered directly to the brain via spinal fluid.
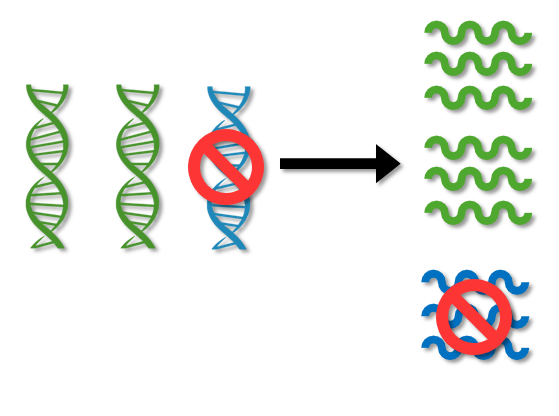
ASOs are designed to silence the extra gene that causes overproduction of APP
Vaccine Immunotherapies
Vaccines train the body’s “security team” to patrol the factory floor, identifying and removing harmful Aß clumps and plaques. The security team inspects the assembly line for faulty parts and clears them out early, while also recognizing and breaking down larger blockages that have already formed. In this way, vaccines act as both preventative measures and cleanup crews.
The ABATE study, sponsored by AC Immune, is currently seeking to determine the safety, tolerability, immunogenicity, and effects of vaccine immunotherapy ACI-24.060 in adults with Down syndrome. This vaccine candidate is delivered like any other vaccine, such to protect against flu, by injection into a muscle.

Vaccines are designed to train the immune system to identify and remove harmful forms of amyloid beta from the brain
Antibody Immunotherapies
Antibody treatments are like specialized “cleaning crews” that step in when harmful clumps of Aß plaques have already built up. These crews target the stubborn blockages, breaking them down and removing them from the factory floor, much like clearing a clogged assembly line to restore functionality.
Commercially known as Leqembi (sold by Eisai and Biogen) and Kisunla (sold by Lilly), anti-amyloid antibodies are currently approved by the FDA for use by neurotypical adults with early Alzheimer’s disease. The upcoming ALADDIN study will be the first investigation of the antibody Kisunla in a cohort of people with Down syndrome.

Antibodies identify, break down, and remove amyloid plaques
These therapies are still being studied, and researchers are working to understand how effective and safe they are for people with Down syndrome-associated Alzheimer’s disease (DS-AD).
While the treatments show promise, it’s important to remember that the goal of these studies is to gather knowledge, not to promise immediate results or cures. Each study is a step toward better understanding how to support brain health in Down syndrome and prevent or delay Alzheimer’s disease.
Families should always consult their loved one’s doctor when considering clinical research participation.
HERO Study (study ended August 2025) | ABATE Study | ALADDIN Study | |
| Study Sponsor | Ionis Pharmaceuticals | AC Immune | Alzheimer’s Clinical Trials Consortium – Down Syndrome (ACTC-DS) |
| Therapy Being Investigated | ION269 | ACI-24.060 | Donanemab (Kisunla, by Lilly) |
| Type of Intervention | Antisense Oligonucleotide | Vaccine Immunotherapy | Antibody Immunotherapy |
| Aim of the Study | To evaluate the safety and tolerability of ION269 in adults with Down syndrome | To assess the safety, tolerability, immunogenicity, and pharmacodynamic effects of ACI-24.060 in adults with Down syndrome | To evaluate the safety, tolerability, and efficacy of Donanemab in adults with Down syndrome |
| Phase | Phase 1b | Phase 1b/2 | Phase 4 |
| Has this therapy been studied in Down syndrome previously? | No | No, but AC Immune conducted a Phase 1 study in Down syndrome with an earlier version of the vaccine | No, approved for treatment of early Alzheimer’s disease but not tested in Down syndrome individuals yet |
| Is the study Placebo-Controlled? | No, not Placebo-Controlled | Yes, Placebo-Controlled | Yes, Placebo-Controlled |
| Method of Delivery | Lumbar Puncture | Intramuscular Injection | Intravenous Infusion |
| Enrollment Criteria | People with DS between ages of 35 and 55, who do not have dementia, have a study partner, and have evidence of amyloid pathology. | People with DS between ages of 35 and 50, who do not have dementia and have a study partner. Participants between ages 35 and 39 require evidence of amyloid pathology. | People with DS between ages of 35 and 50, who do not have dementia and have a study partner. |
| Requires MRI/PET scans? | MRI + PET | MRI + PET | MRI + PET |
| Expected Enrollment | 30 | 80 | 60 |
| Participation Duration | ~1 year | 2 years | 2 years |
| Active Locations | US, Spain | US, UK, Spain | US |
| Date of Study Completion | 2027 | 2026 | 2027 |
| Clinicaltrials.gov link | NCT06673069 | NCT05462106 | NCT06911944 |
Overview of Investigational Therapy Types:
Overview of Methods of Delivery:
Defining Study Phases:
Placebo Controlled:
Randomization:
Blinding:
