
Did you know that the lifetime risk of developing Alzheimer’s disease by age 65 in people with Down syndrome is over 90%?
*Fortea 2021 Alzheimer’s disease associated Down syndrome — a genetic form of dementia.

Versus: the lifetime risk for woman in the general population is a 21% chance, and the lifetime risk for men in the general population is a 12% chance of developing Alzheimer’s disease by age 65.
*2024 Alzheimer’s Disease Facts and Figures Special Report: Alzheimer’s Assocation
Alzheimer’s disease (AD) is the leading cause of death of adults with Down syndrome. Alzheimer’s disease is an urgent medical concern for the Down syndrome community. By working with the Down syndrome community, scientific researchers, and the life science industry to develop evidence-based therapies and treatments, LuMind IDSC is focused on combatting the onset of Down syndrome related Alzheimer’s disease (DS-AD).
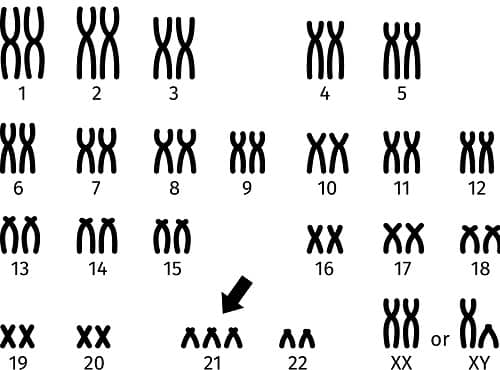
Human chromosomes usually occur in pairs. Neuro-typical individuals are born with 23 pairs of chromosomes, while people with Down syndrome are born with three copies of Chromosome 21. The Amyloid Precursor Protein gene (APP) that produces amyloid protein is located on Chromosome 21.
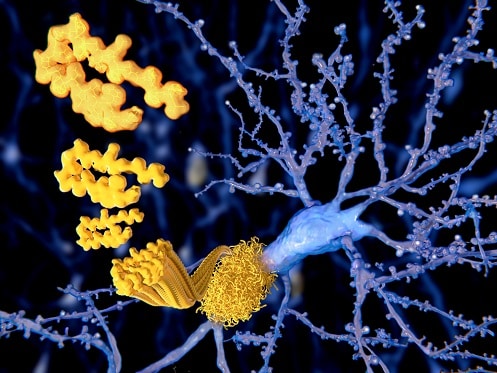

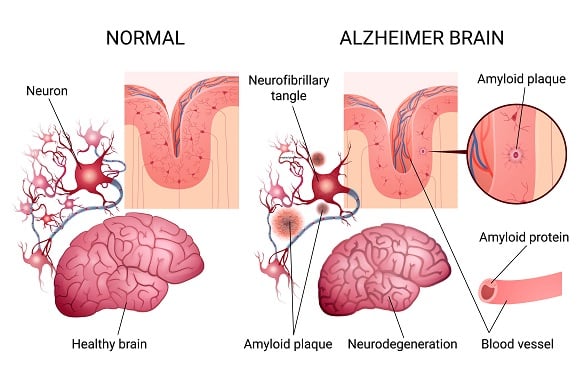
On July 7, 2023, the U.S. Food & Drug Administration (FDA) granted full approval for Leqembi, a drug that slows the progression of Alzheimer’s disease.
Leqembi, which was co-developed by biopharmaceutical companies, Eisai and Biogen, is approved to treat patients in the early dementia stage of Alzheimer’s disease who also have “confirmed presence of amyloid beta pathology” in the brain. Read more about Leqembi’s conditional approval in January 2023 here.
For the 1.5 million people in the early stages of Alzheimer’s disease, the FDA decision is a reason to celebrate. Not only is Leqembi the first anti-amyloid drug to receive full approval from the FDA, it has also demonstrated better and more consistent clinical trial results than its predecessor, Aduhelm™, which was conditionally approved for use in June 2021.
While this breakthrough is a milestone in treating Alzheimer’s disease, there are still questions that remain for our Down syndrome community. You can read our official statement about Leqembi online. Additionally, we have included 5 Updates on Down Syndrome Community Access to New Alzheimer’s Drugs online for your reference.
Helping people who are “on the path” to Alzheimer’s disease is why new medical tests called biomarkers have been developed. Up until recently, scientists did not have biomarkers to measure changes in the brain so most of the drug development efforts were focused on people who were already showing symptoms caused by Alzheimer’s disease.
Because there are still so many unknown factors about how to repair the damage done by Alzheimer’s disease, many scientists are now focused on disease prevention. In fact, because biomarkers can predict and measure neurological change over time, much of the Alzheimer’s research field is moving toward prevention and enabling strategies for early treatment of symptoms.
If you want to find out more about current scientific advances, follow our Research Spotlight blog.
Dr. James Hendrix and a committee of researchers from the Alzheimer’s Association, recently published Committee on High-quality Alzheimer’s Disease Studies(CHADS) Consensus Report, which will guide the protocol development and conduct of clinical trials in Alzheimer’s disease research.
This report may further provide a platform for the development of education materials that may help guide appropriate clinical trial participation decisions for potential trial participants and the general public.
