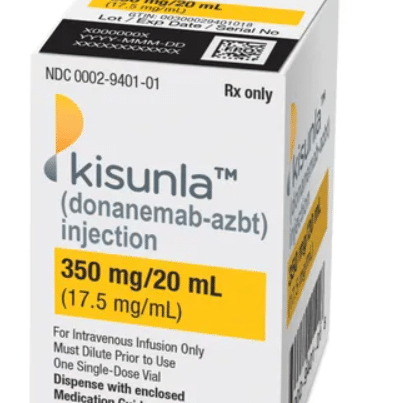
FDA issues approval for new Alzheimer’s drug Kisunla; Down syndrome community is optimistic
FDA issues approval for Kisunla, new Alzheimer's drug; Down syndrome community optimistic On Tuesday, July 2, the U.S. Food and Drug Administration (FDA) announced the approval of Kisunla (donanemab), an…